This post shows the concept of pH and its influence on the nitrogen cycle. This is a continuation of the previous post about Environment.
To know what is nitrogen cycle, click on the following button.
What is pH?
It’s the abbreviation of potential of hydrogen and a measure in logarithmic scale, that indicates the degree of acidity or alkalinity in a solution. When higher the quantity of hydrogen ions (H^{+}) dissolved in aqueous solution, more acid is the substance.
Calculation
The pH is the negative logarithm of H^{+} in mol/l on 10 base.
pH=-log[H^{+}]
[H^{+}]=10^{-pH}
This equation shows that each number has 10 times more H^{+} concentration in aqueous medium than the higher number. For example, a substance with pH equal to 5 is 10 times more acid than a solution with pH 6.
pH influence on the nitrogen cycle
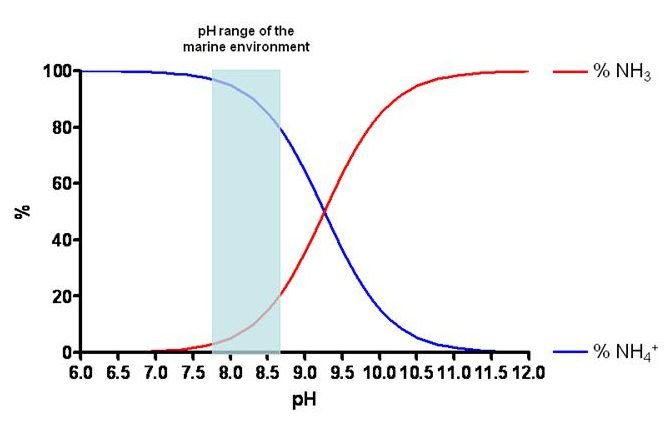
Land plants use ammonia to synthesize aminoacids. In the aquatic environments, ammonia (NH_{3}) is toxic for fishes. This molecule can cross the fishes’ gills. When it’s inside the gill, can convert in ammonium ion (NH_{4}^{+}), this conversion damages the cells.

Furthermore, the excess of NH_{3} harm the corals’ skeleton, making them less dense. However, the NH_{4}^{+} isn’t toxic for fishes, because it can’t enter on gills.
Le Chatelier principle on nitrogen cycle
The ammonia forms a chemical balance with ammonium ion, shown by the following reversible chemical reaction.
NH_{3}+H_{2}O\rightleftharpoons NH_{4}^{+}+OH^{-}
A chemical system is on balance when the reactions’ speed on both sides of the reversible equation are equal and when the concentration of involved substances are constant. Le Chatelier principle claims that, when the balance of a chemical system is disturbed by an external factor, this system readjusts itself to stay in a new balance. In the chemical system above, which is part of nitrogen cycle, when lower the water’s pH, bigger the production of NH_{4}^{+}.