In this post, I will make an unboxing of a hydrogen and solar energy kit. This kit serves to make experiences with solar energy and a hydrogen fuel cell.
Materials
These are the kit’s materials. Blue block is the hydrogen cell, white big parts are the support and the white box with wires is to 2 AA batteries.
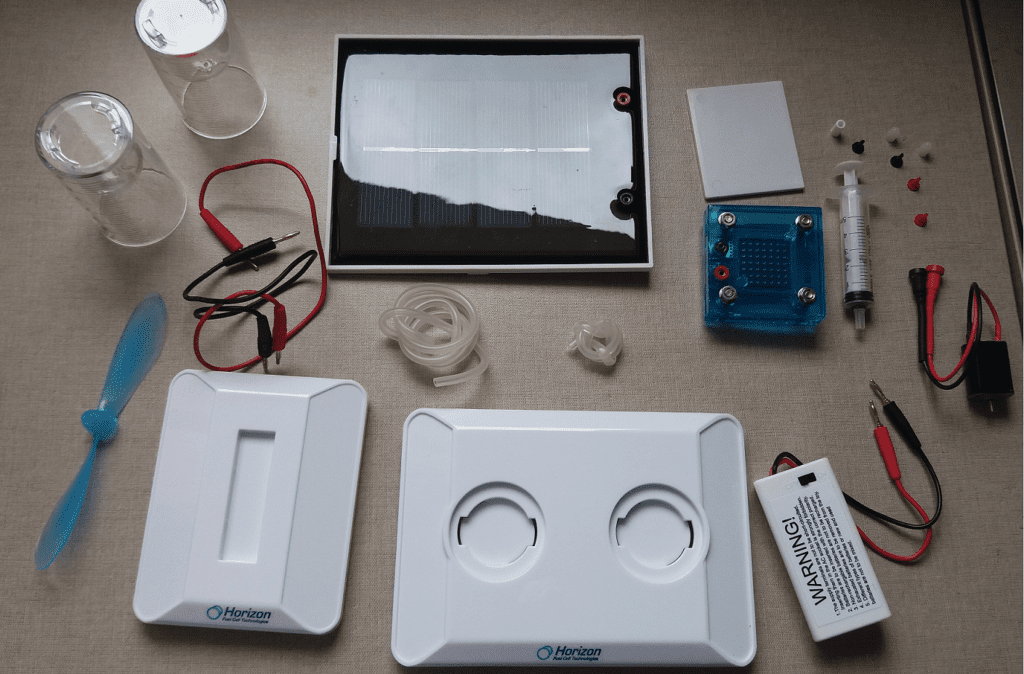
Also has a CD with instructions.

Following the assembly guide
I assembled the kit following the assembly guide on paper. The cell in the support.

I put rubber hoses cut and pins in the cell to inject water.
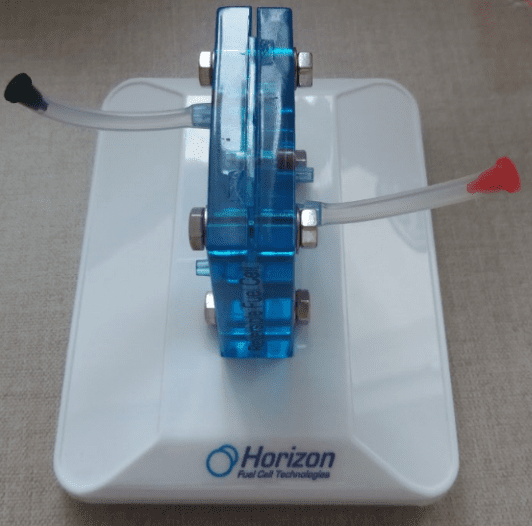

Plastic cylinders in the support.

It is necessary to use distilled water to put in the cell.

Put distilled water in the cylinders and hydrogen cell.

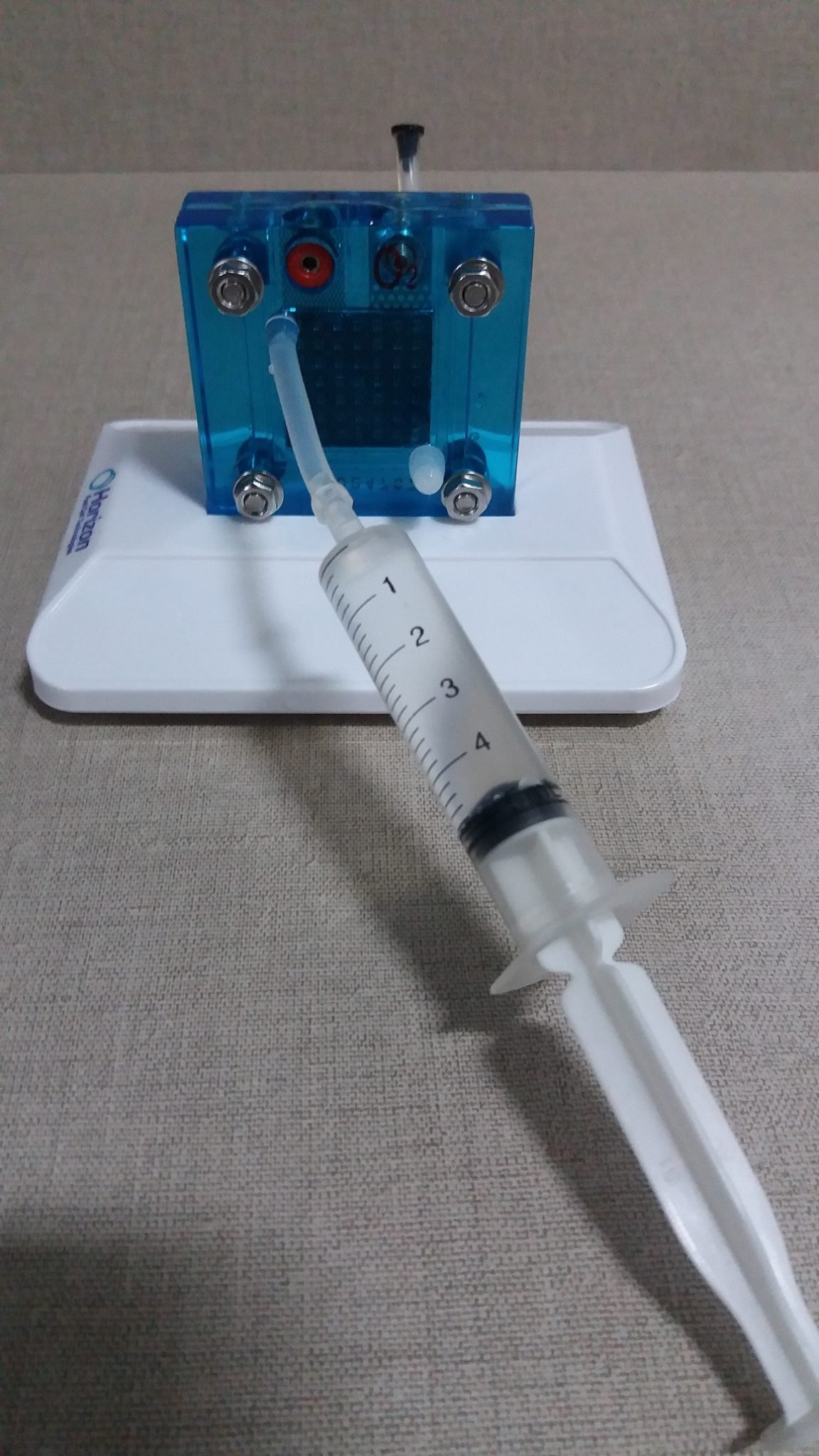
Are needed two hoses of equal size.

The module was assembled.

The next step is to supply the hydrogen and solar energy kit with an electric voltage, you can use a solar panel or batteries. But since there wasn`t Sun at the time I build it, I used 2 AA rechargeable batteries in a series. To know how solar panels work click on this button.
 Solar panel (Part 1)Click here
Solar panel (Part 1)Click here
This is the video showing the supply of hydrogen cell and after this same cell supplying a DC motor.
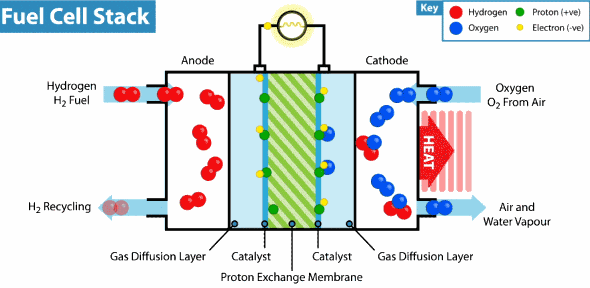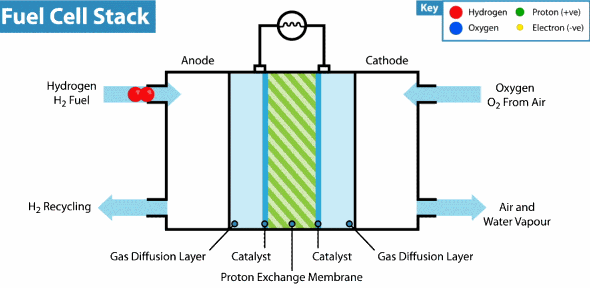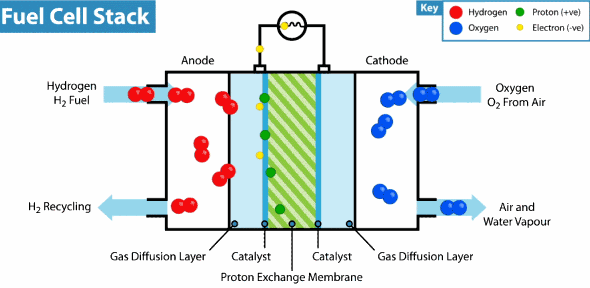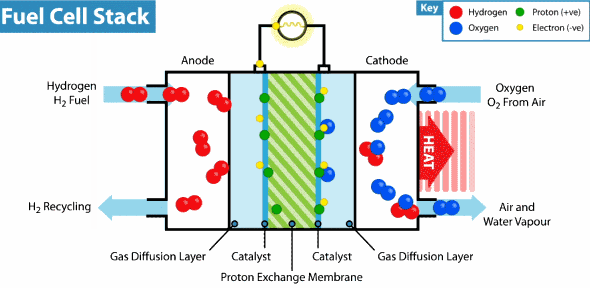
How does the hydrogen fuel cell work?
Now I will explain how the cell from hydrogen and solar energy kit works. A hydrogen cell converts the hydrogen and oxygen gases into electrical energy by a reaction known as electrolysis. The cell has cathode and anode electrodes, which have channels to a flux of water, oxidizer, and non-used fuel and have backing layers.
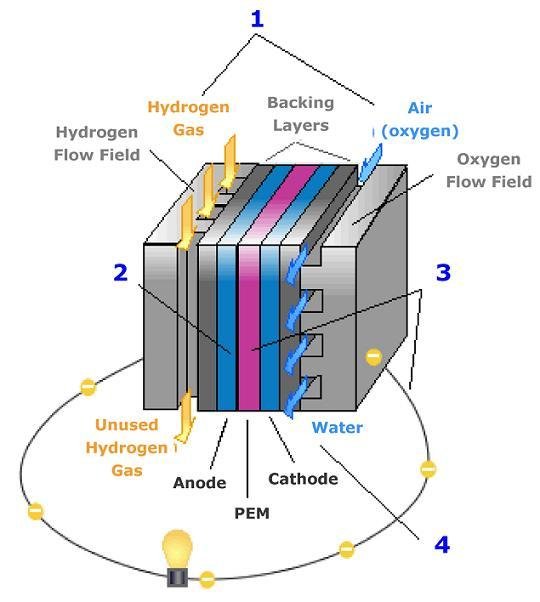
You can make a stack of cells.

Between the electrodes, there is a polymeric electrolytic membrane that has the function of transferring protons from a side to another. The electrolyte material can be a polymer with H_{3}O^+, phosphoric acid (H_{3}PO_{3}), molten carbonate (CO_{3}^{2-}) or zirconia (ZrO_{2}). Each material has advantages, disadvantages, and applications. The membrane in this kit is made of polymer because the temperature operation is from 20°C to 120ºC, other materials require higher temperatures.
When I was supplying the cell with batteries in the first part of the video, I was making this chemical reaction.
2H_{2}O\rightarrow 4H^+ + 2O^{2-}
4H^+ + 4e^- \rightarrow 2H_{2}
2O^{2-}\rightarrow O_{2} + 4e^-
2H_{2}O\rightarrow 2H_{2} + O_{2}
The bubbles which were going to the recipients were of hydrogen and oxygen gases.
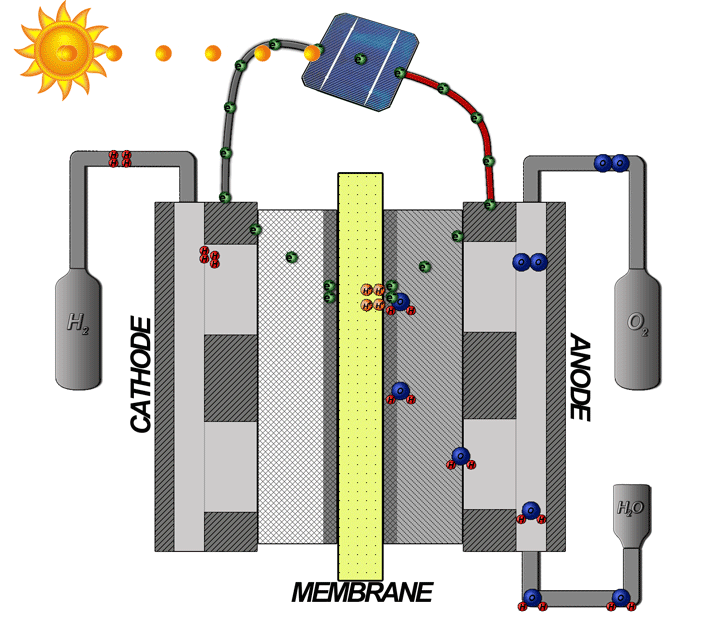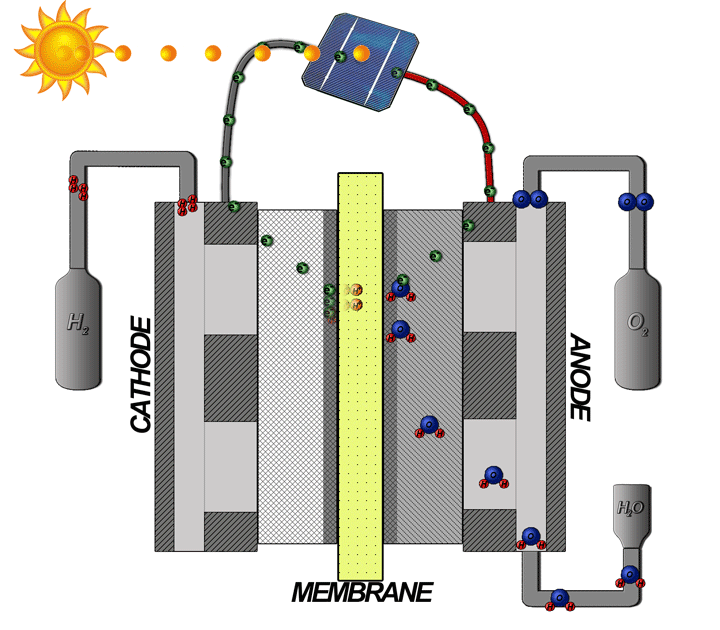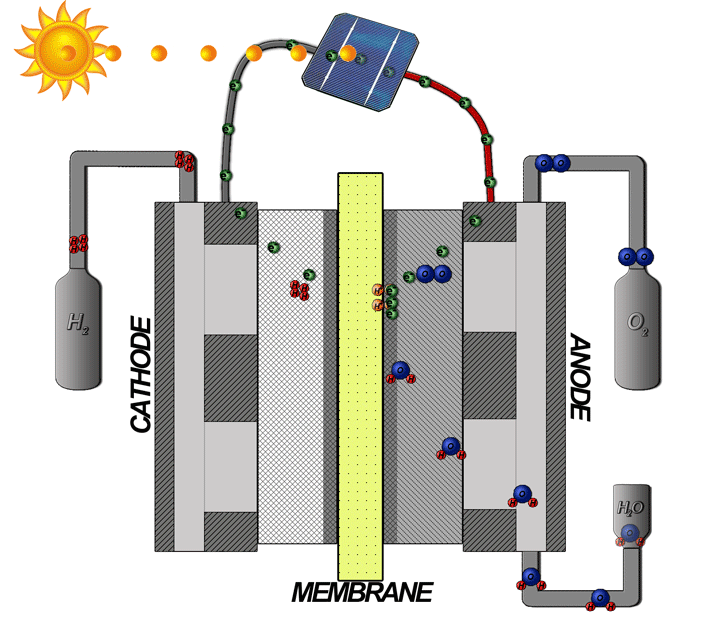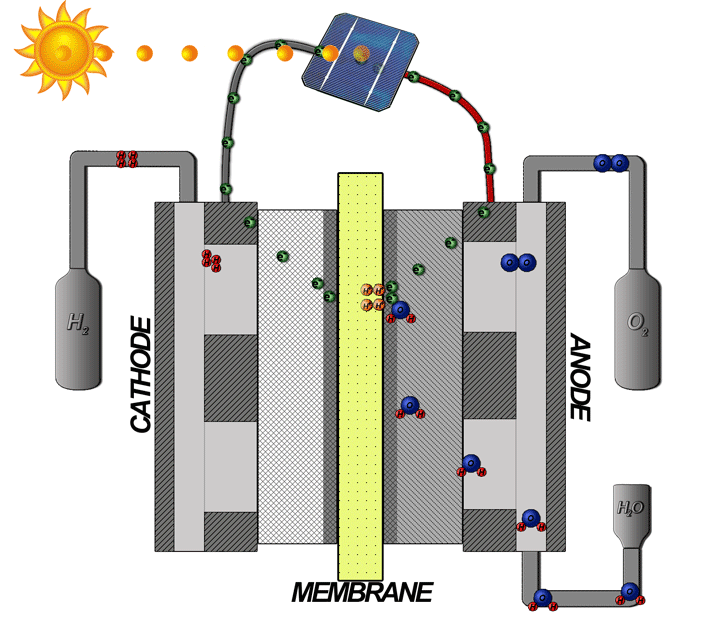
When I supply the motor with the cell I make the inverse way, transforming the hydrogen and oxygen gases in water and electrical energy to supply the motor.



Click on GIF in the images.