A new sodium-sulfur battery design can become a cheaper and safer alternative to lithium batteries.
Currently, lithium is the most used element in batteries. To know more, click on the link below.
Lithium: properties, extraction and applicationsClick here
Source: TechXplore
Common battery materials, like lithium, can be prone to disadvantages like overheating and material sourcing issues, leading to safety risks and higher costs.
Now, researchers from China have revealed a new battery design that may offer a better alternative to lithium. The new study, published in Nature, describes a sodium and sulfur-based, anode-free design offering a high voltage. The sodium–sulfur (Na–S) batteries are a promising alternative to lithium-based batteries due to sodium’s abundance and potential for high energy storage.
This is not the first battery design to combine sodium and sulfur. However, prior Na-S batteries faced issues limiting their practicality. Some Na–S batteries used S/Na2S chemistry, which was limited by low voltage and high sodium metal requirements. And despite the high-valence sulfur redox (S0/S4+) providing higher voltages of around 3.6 V, researchers had not found a way to create this reaction at room temperature due to energy barriers.
The study authors write, “The S/Na2S conversion reaction at the cathode yields a limited discharge voltage of less than 1.6 V versus Na/Na+, which is much lower than those achieved by the cathodes of current Li and Na batteries. Also, the use of substantial Na metal at the anode, generally exceeding those of conventional Li and Na batteries by tens of times, undermines cost-effectiveness and safety, while sacrificing the available energy and power densities.”
The key to the new design was “unlocking” the high-valence S0/S4+ redox chemistry to create the high-voltage anode-free Na–S batteries at typical room temperatures. The new design consists of a S8 cathode, an aluminum (Al) foil anode current collector, a glass fiber separator and sodium dicyanamide (NaDCA) in a non-flammable chloroaluminate electrolyte. The new battery offers a discharge voltage of 3.6 V.
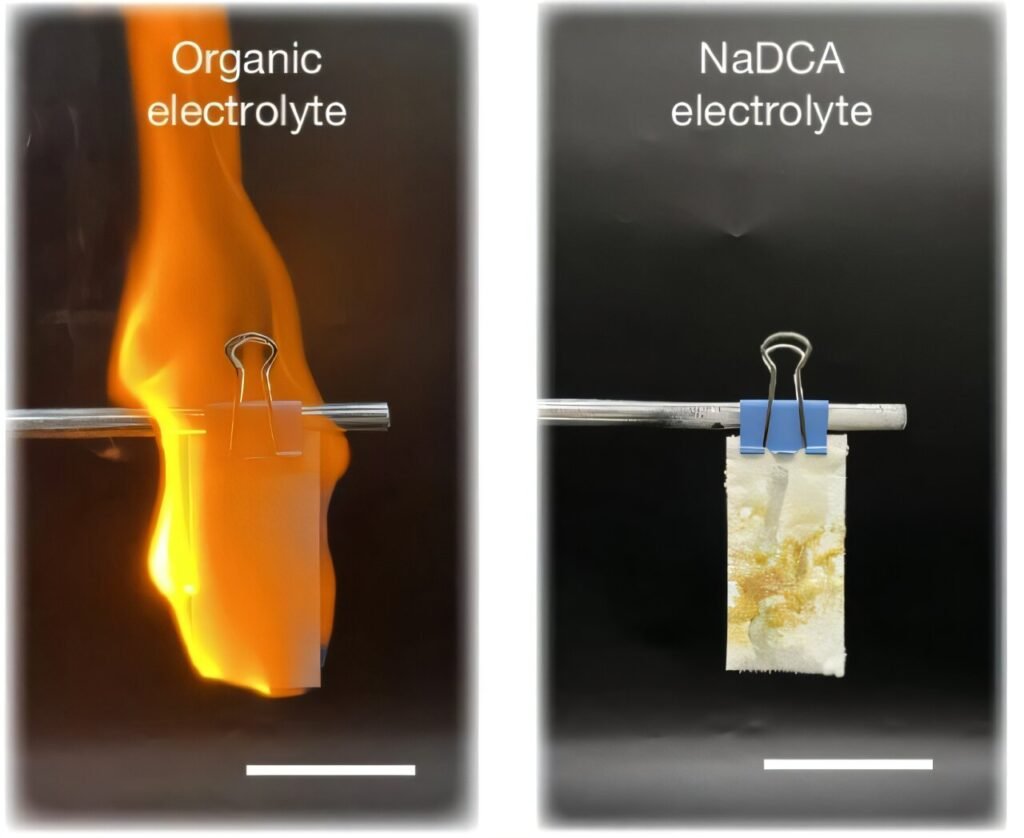
“Mechanism studies show that the dicyanamide anion in an optimized chloroaluminate electrolyte plays a crucial role in unlocking the S/SCl4 cathode chemistry and also enhancing Na plating/stripping reversibility at the anode, which together realize high-voltage anode-free Na–S batteries with excellent electrochemical performance and practicability,” the study authors say.
The new design achieved maximum energy density of 1,198 Wh/kg, a discharge capacity of 715 mAh g−1 and power density of 23,773 W/kg. The team says that incorporating a Bi-COF catalyst in the cathode further increased discharge capacity to 1,206 mAh/g and energy density to 2,021 Wh/kg.
The team also estimates that the cost of the new design is far lower than current alternatives. At $5.03 per kWh, it is one to two orders of magnitude lower than current Na batteries. Sodium’s abundance and lower extraction impact make it a more sustainable material than lithium, as well.
Although the team says the new batteries are promising for practical energy storage applications, some limitations still remain before these new materials can be used practically. One issue to be addressed is that the AlCl3/SOCl2-based electrolyte is corrosive and challenging to handle, requiring further study. In addition, the air stability is short-term, meaning long-term or large-scale exposure safety is uncertain.



