Nanomachines are molecular mechanisms which execute a function when receive a stimulus. Today’s subject is an introduction with examples.
Natural nanomachines
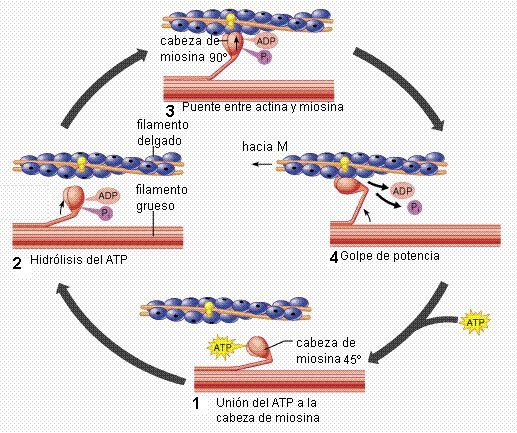
In nature, exist a lot of nanomacines which make many functions. An example is myosin. Converts chemical energy in ATP (Adenosine triphosphate) molecule in mechanical motion over actin to muscle contraction.
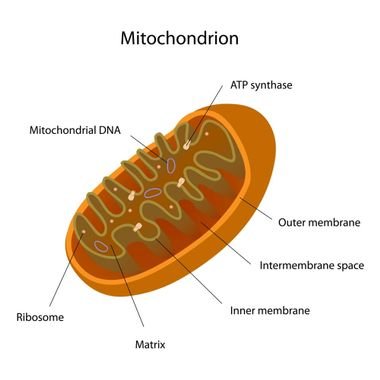
Another example is the enzyme F0F1-ATP synthase, located in mitochondria. Its function is to synthesize ATP, molecule which provides energy for cells, with ADP (Adenosine diphosphate) and inorganic phosphate.
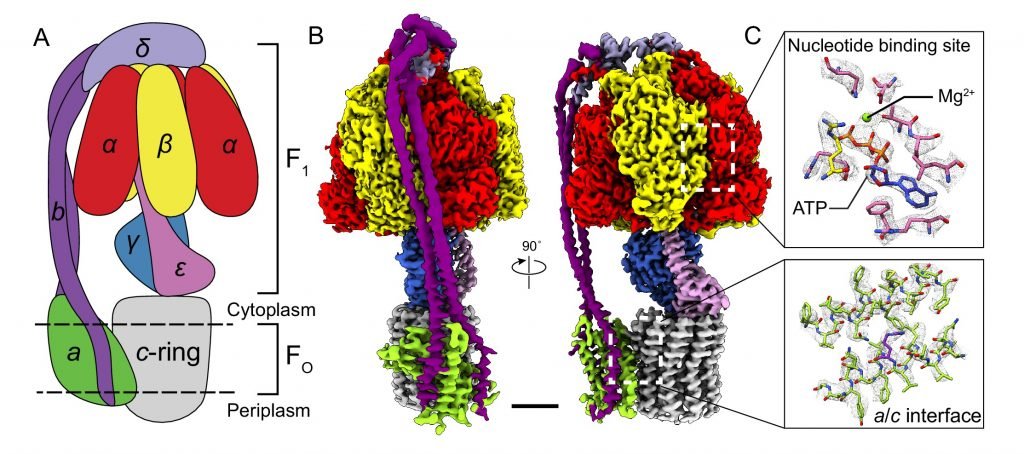
Protons from intermembrane space, where there is a great concentration of hydrogen ions, induce rotation in c-ring, the later moves Y and ε subunits, making ATP synthesis in the matrix. The F0 part is in inner membrane. This machine is extremely efficient.
Artificial nanomachines and some examples
Many of artificial nanomachines are inspired in macro size machines like gears, propellers, switches, etc. The great majority is made of organic molecules with benzene rings, hydrogen, oxygen. Some use metallic ions. These machines’ performance depends on electrostatic interactions and intermolecular forces. The stimulus for molecular machines can be electric, luminous, chemical, or thermal. However, they still are much more primitives than natural ones. Remembering that in nanometric scale, physics laws are very different from macro scale.
Catenane

Formed by two mechanically intertwined shaped-ring polymer molecules. Can’t separate the molecules without break a covalent bond. The first catenane was created in 1983, with two rings intertwined around a copper ion. With thermal or electrochemical stimulus, depends on the polymer, rings can make motions of rotation, translation around another ring’s axis, or inclination.
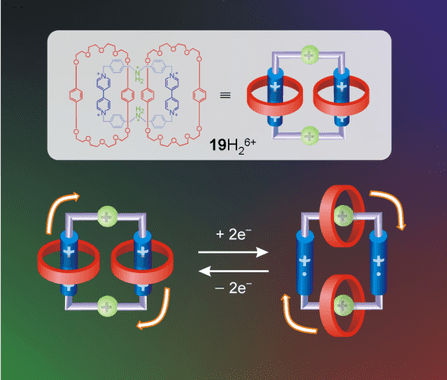
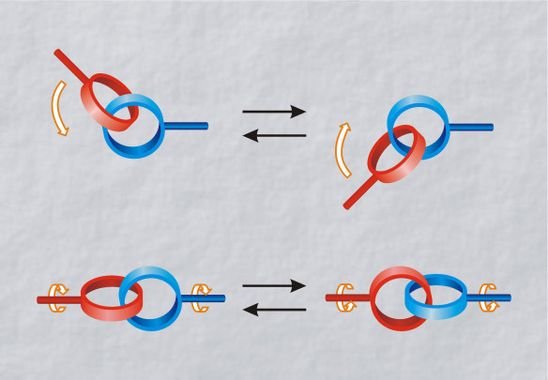
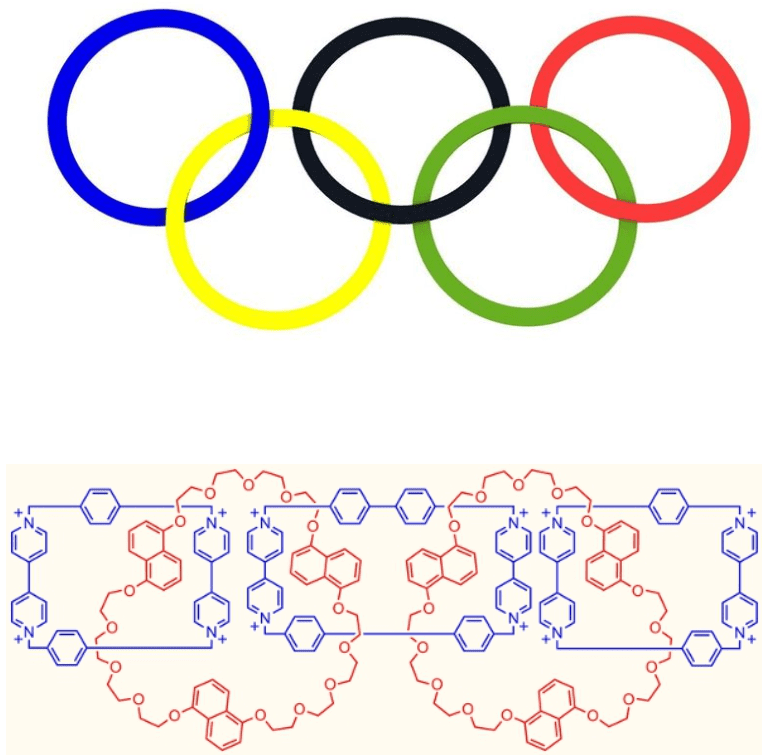
It’s possible to assemble many rings and create a long chain polycatenane.
Rotaxane
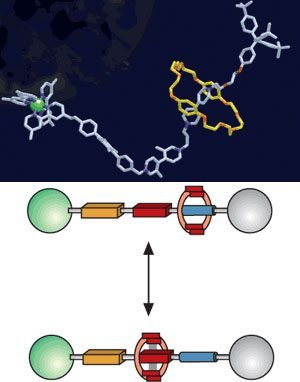
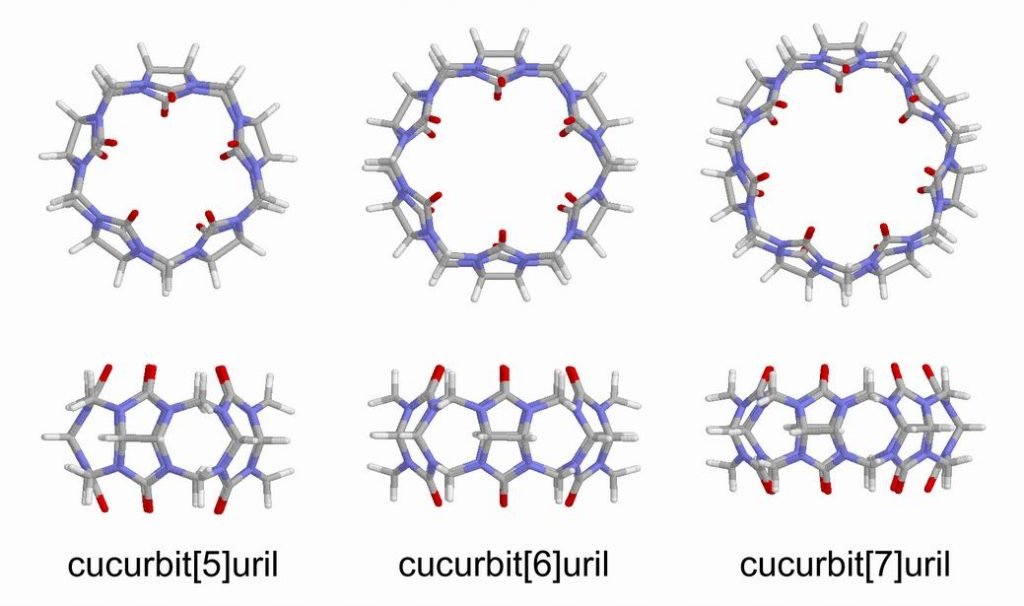
It’s composed of a linear molecule, with bulky chemical groups in extremities. This is threaded by another molecule in a ring shape. The ring-shaped macrocyclic molecule is called Cucurbituril or CB. The number between square brackets indicates ring shape saw from above. For example, Cucurbit[5]uril has a pentagon shape. Are composed of many atoms for CHON group (carbon, hydrogen, oxygen, and nitrogen).
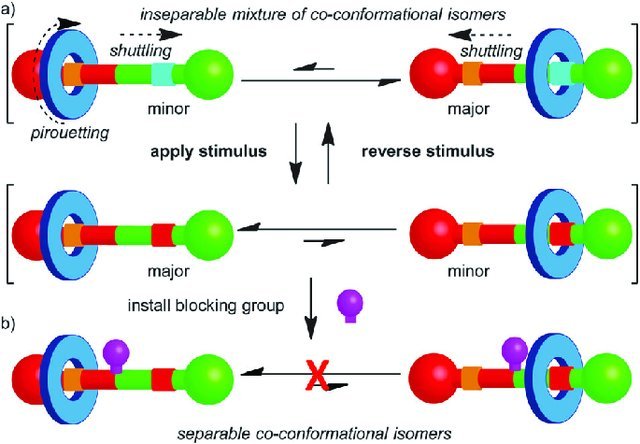
With a chemical, electrochemical or luminous stimulus, ring changes position in the linear molecule. It can be used as a two-state switch.
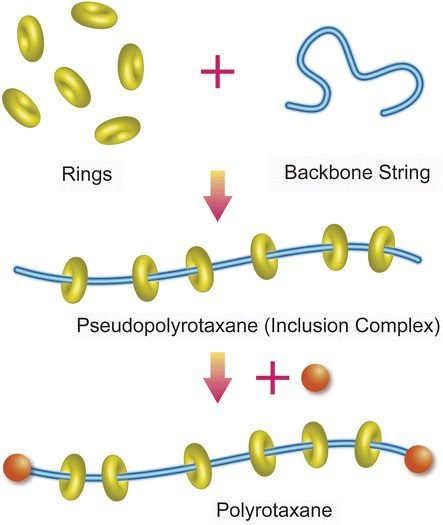
Rotaxane can have more than one ring and form polyrotaxane. If you take out bulky groups from extremities, it becomes pseudorotaxane.
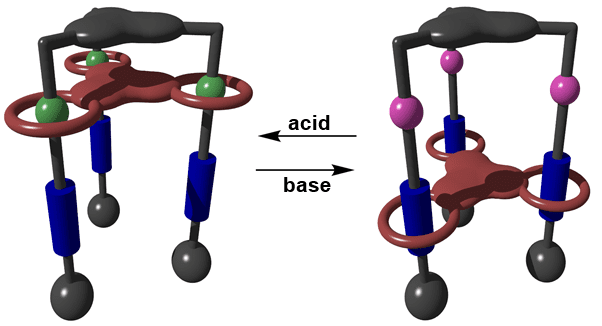
Rotaxanes can be used to build nanolifts. Transporting molecules for some nanometers.
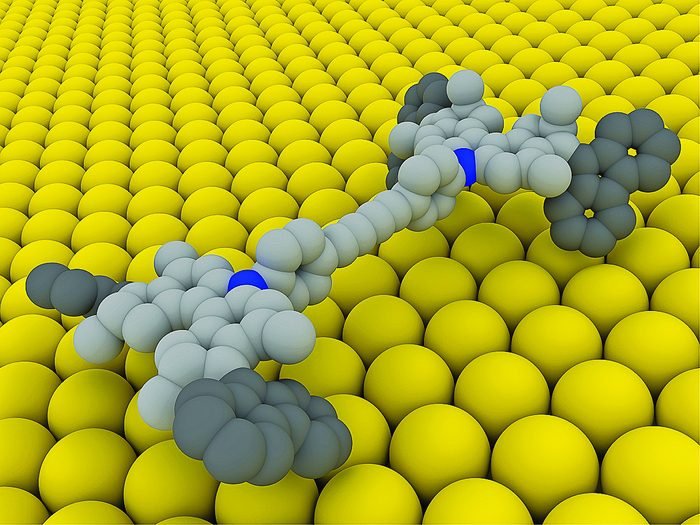
Nanocar

A nanocar has only one central chassis and four “wheels” linked to the axle. Light incidence excites some electrons, causing an isomerization reaction, structure change without alteration of the chemical formula. The double covalent bond linking the wheel to axle becomes single. Some nanocars are moved by electrons from tunneling microscope to make isomerization reaction, the molecular structure allows rotation in only one direction. All nanocars which currently exist move in a metallic surface, usually copper or gold.

Exist nanocars with fullerene wheels, which roll in high temperature surfaces and don’t have an engine.
Nanomachines will be used to build nanorobots, which will have many applications.