Today’s subject is Raman spectroscopy. A very used technique in physics, chemistry, and biology to analyze materials’ molecular structure.
The physical principle behind Raman spectroscopy
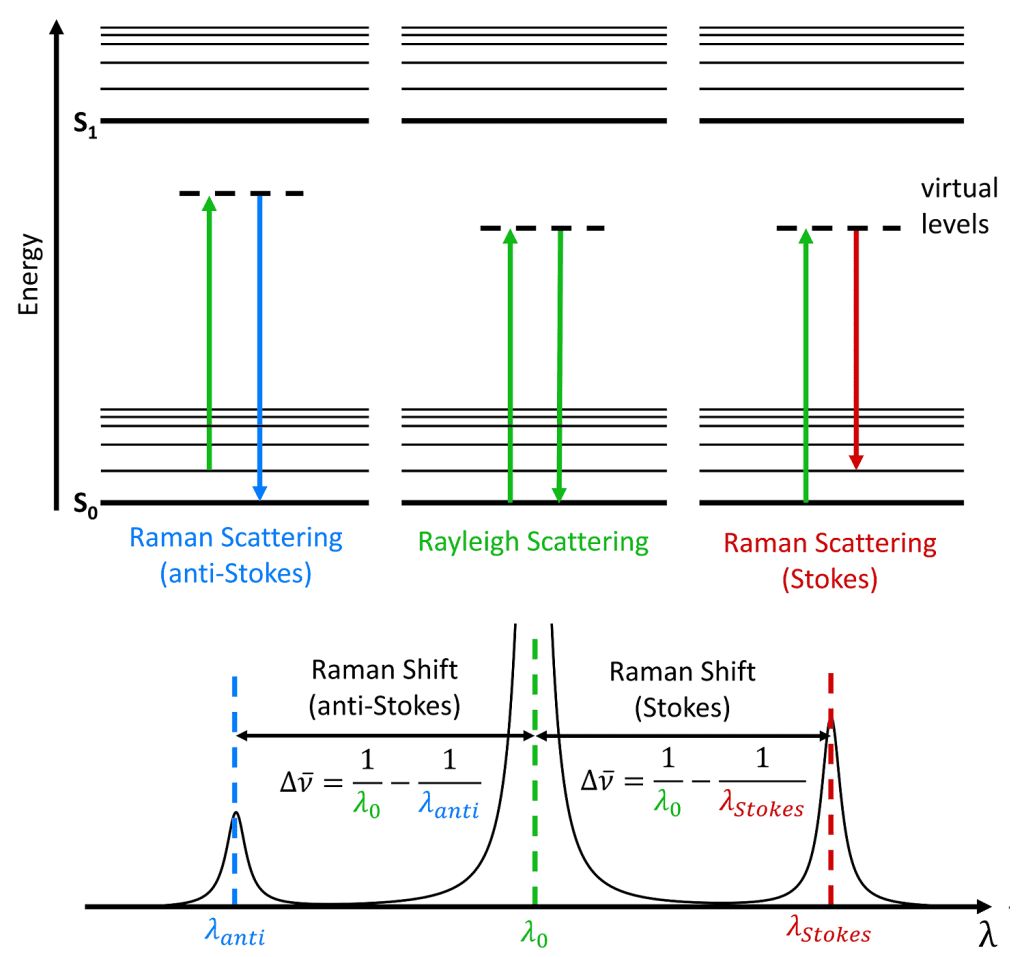
When monochromatic light interacts with a molecule, the photons (light elementary particles) are dispersed. The majority of dispersed photons have the same wavelength \lambda of incident light, it’s called Rayleigh, or elastic, scattering. A minority of photons is scattered with different wavelengths, this phenomenon is called Raman scattering or shift.
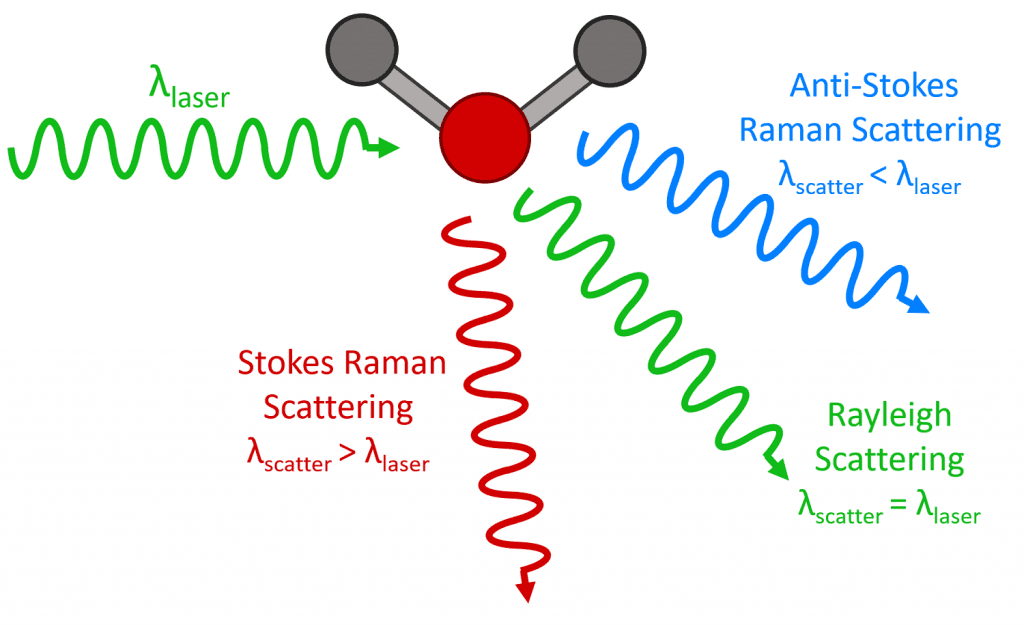
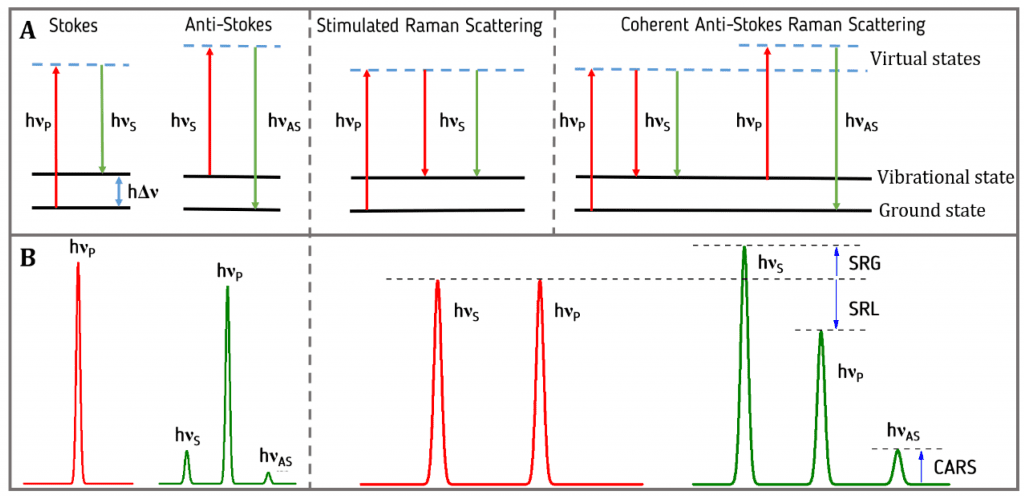
How molecules scatter the light? When an electron interacts with an incident photon, the electron gains energy and passes to a virtual state with more energy. This virtual state is unstable, a photon is emitted, and the electron returns to previous energetic state. This is Rayleigh scattering. A minority of electrons don’t go back to the previous level when emits a photon, they stay in a vibrational state. If the light in the output has a lower frequency, the shift is called Stokes. If frequency is higher, the shift is anti-Stokes.
The absorbed and emitted energy E by the electron follows the equation:
E=h\cdot \nu
Where \nu is the frequency in Hz and h is the Planck constant, whose value is 6,6261\cdot 10^{-34}J\cdot s.
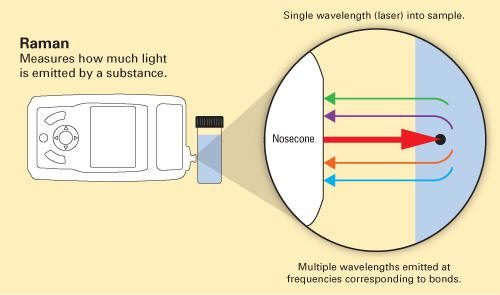
Raman microscope

The instrument used to identify the material. Every Raman microscope has a high intensity solid state laser (Excitation Light), usually, the lasers’ wavelengths are 532, 85, and 1064 nm. This light passes through an objective lens before arrives the sample.

The monochromatic beam is scattered by the sample, light with elastic scattering is filtered, while light with inelastic scattering is diffracted. An array of CCD sensors detects and amplifies the light, transmitting the signal to be analyzed by software in a computer.
Polarizability
Each molecule has a different Raman shift, like a signature. The scattering depends on polarizability, which is the measure of electron cloud distortion in the presence of an electric field.

Disadvantages and limitations
- Can’t be used in metals or alloys.
- The Raman shift is weak, therefore, has low sensitivity. Can be corrected with some techniques.
- Can’t be used in fluorescent materials, because they interfere with detected light.
- Samples that absorb too much light increase temperature, can cause changes in molecular structure, and ignite.
Exists another spectroscopy method called FTIR, but will be subject to another post.
Some practical applications
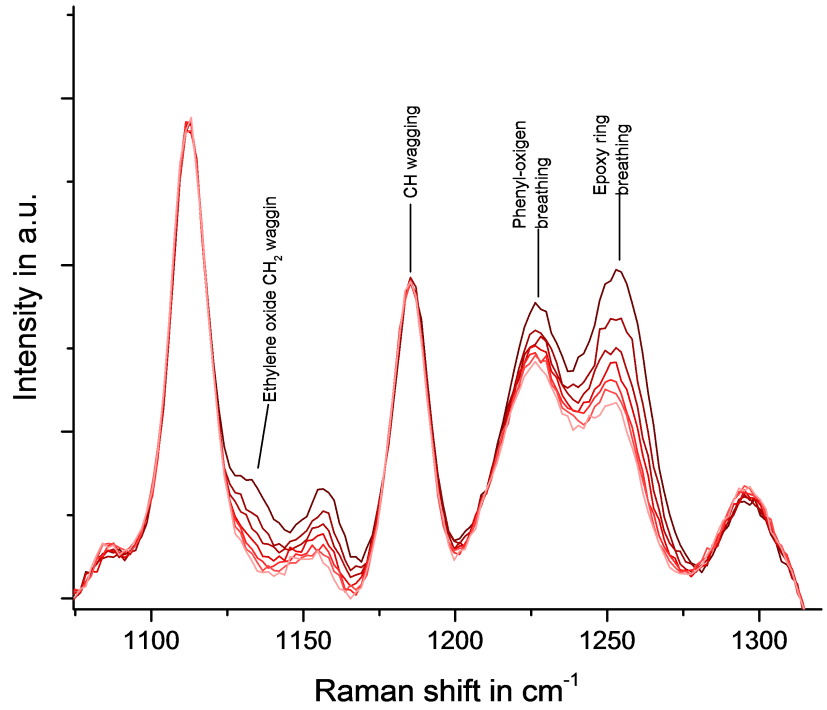
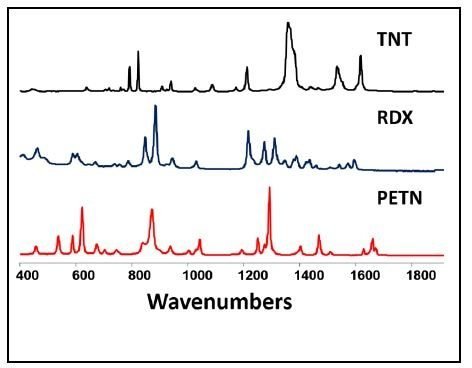




[…] laser is used in eye surgeries, due to high precision. In addition to be applied in lithography, Raman spectroscopy, entertainment, holography, high speed printing and forensic […]