This post’s subject is Geiger-Müller counter. An instrument invented in 1908, which identifies radioactive objects and measures ionizing radiation level.
The structure
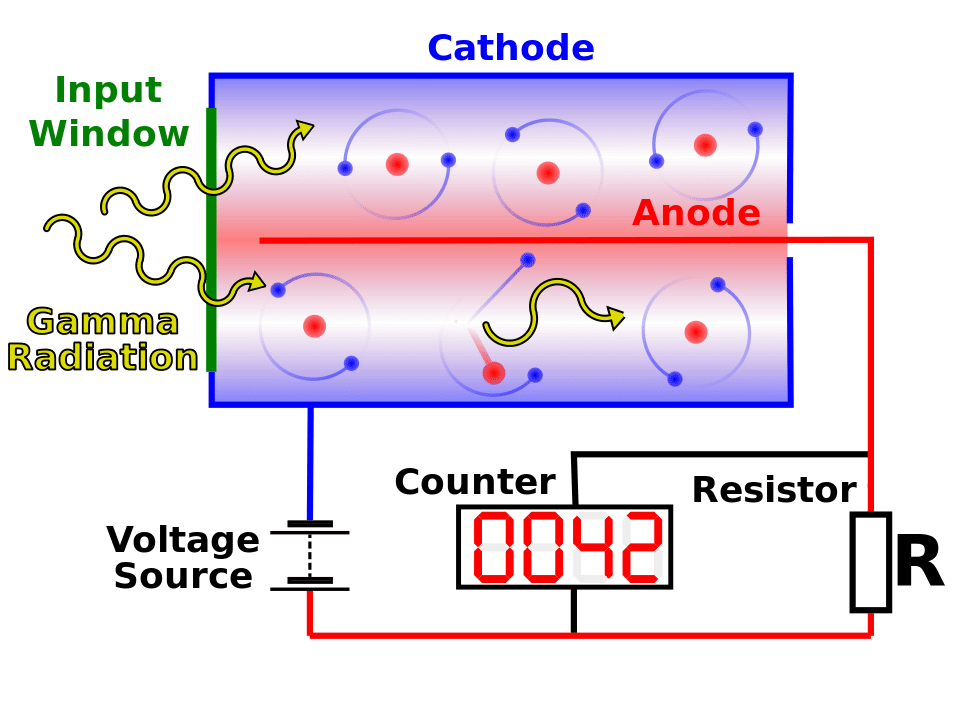
The sensor is a tube made of conductor material, the cathode. Anode is an electrode made of tungsten which is inside the tube. Both are linked to a high voltage power source and a resistor in parallel with an amplifier and digital counter. On the electrode’s opposite side, there’s a thin mica layer, called window, where enters ionizing radiation to inside the tube.

Inside of Geiger-Müller counter tube
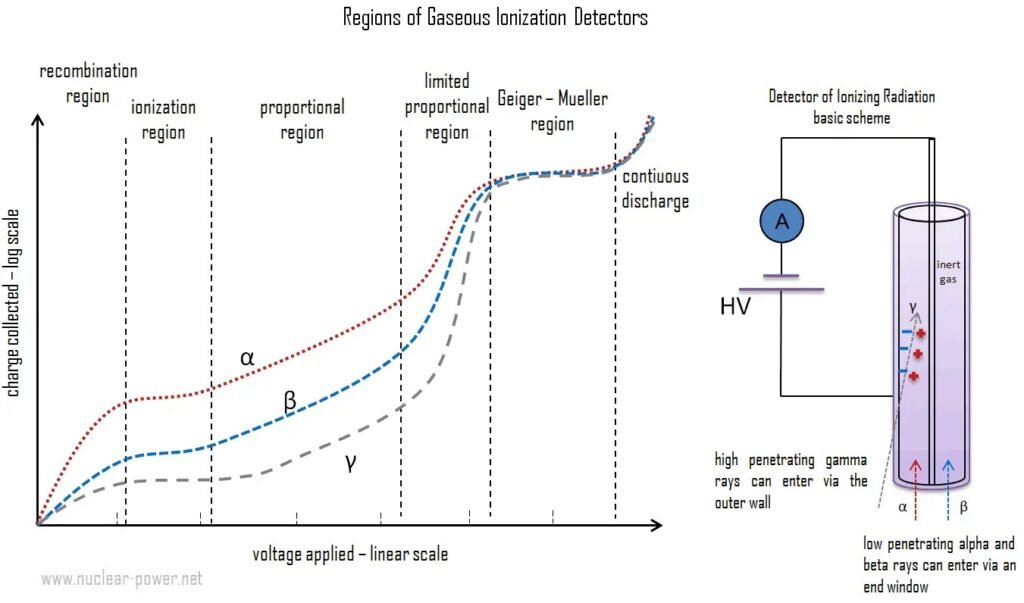
Inside the tube there is a low pressure inert gas, whose composition is usually 90% argon and 10% ethanol or halogen gas. In normal conditions, argon is insulating, however, when alpha (α), beta (β) and gamma (γ) particles enter into the tube, argon atoms are ionized, because they lose electrons when interact with ionizing particles. Electrons are attracted by anode and ions by cathode, due to electric field created by high voltage power source. Consequently, electric current pulses are produced.
Townsend avalanche
When electrons is separated from atom due to ionizing particle, electric field between electrodes speed up electrons to collide with other atoms, producing more ions and free electrons, which will collide with more atoms. Creating Townsend avalanches or discharges in direction to anode electrode.
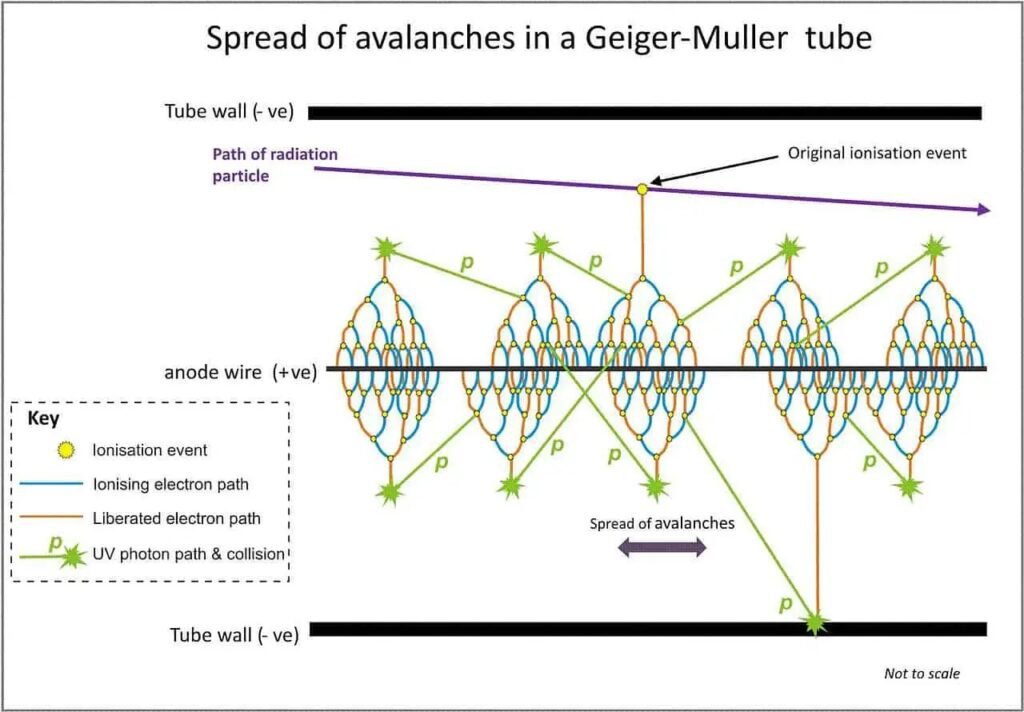
Why does the gas have 10% ethanol? To prevent oscillations and an uncontrolled increase of electric charges and, avoid continuous discharge. Because the avalanche must stop if there’s no incident particles. This gas ionization potential is lower and when an electron or ion interacts with this gas molecule, it’s absorbed or has it’s speed reduced. The organic gas molecules are broken, but electrons are emitted.
Limitations of Geiger-Müller counter
- Can’t distinguish ionizing particles.
- Can’t measure high rate of radiation due to a “dead time”, time that takes to recover from pulses induced by Townsend avalanches.
Some curiosities
- Hans Geiger worked with Ernest Rutherford, the physicists who discovered the atomic nucleus, when he invented the counter. However, only detected alpha particles. In 1928, Geiger and Müller improved the device to detect other particles.
- The Geiger-Müller counter was used to study cosmic rays.
- To detect neutrons, which don’t have electric charge, the gas must be helium-3 or boron trifluoride (BF_{3}) or the tube’s inner wall must be coated with boron.


