The perovskite promises to revolutionize the photovoltaic solar energy. Properties and use on solar energy are this post’s subjects.
Perovskite crystalline structure
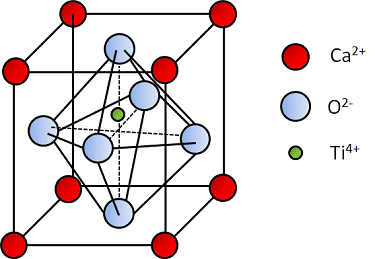
The perovskite mineral is a titanium and calcium oxide, whose chemical formula is CaTiO_{3}. It was discovered in 1839, in Ural Mountains, Russia, by the German mineralogist Gustav Rose. The mineral’s name is a homage to the Russian mineralogist Lev Perovsky.

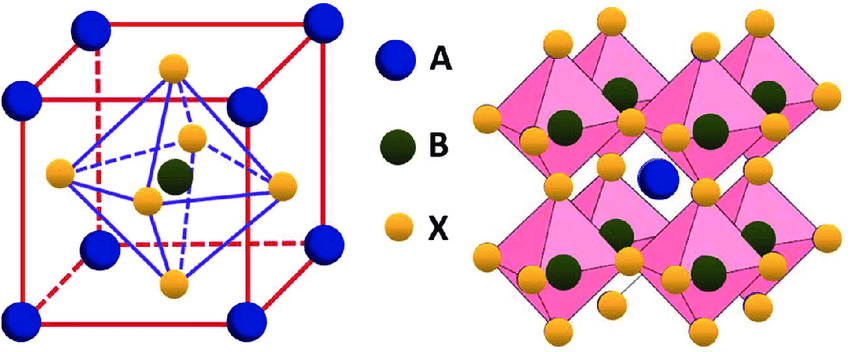
In the mineral’s case, A and B are calcium (Ca) and titanium (Ti), respectively, while X is oxygen (O). Therefore, electric charges from each element are: +2, +4 and -6 respectively, because oxygen is -2 and there are 3 atoms on formula.
Exist other materials with the same structure, which have many applications. Depending on atoms that compose the structure, a perovskite material can show notable electronic and optical properties, which can be useful in optoelectronic components, such as:
- High mobility of electric charge carriers.
- High light absorption coefficient in a wide frequency range.
- Adjustable band gap.
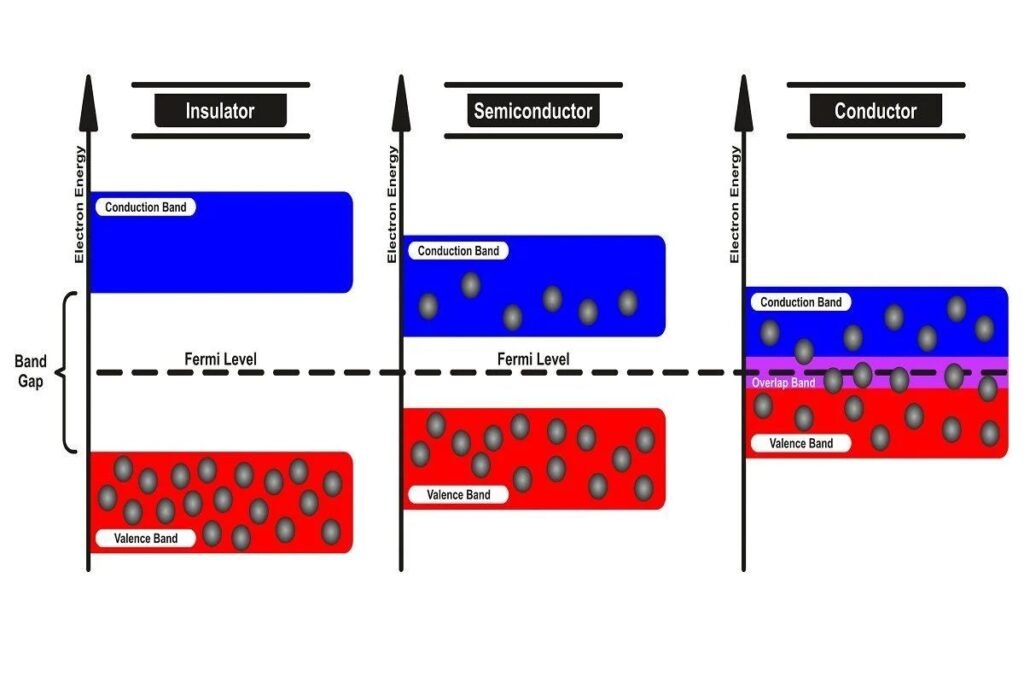
In addition to other properties, such as:
- Superconductivity.
- Giant magnetoresistance.
- Piezoelectricity: capacity to produce electric charge when it receives a mechanical stress.
- Dielectric properties.
- Catalytic properties.
- Ferroelectricity: spontaneous electric polarization without an external electric field.
Perovskite solar cell
One of perovskite that can convert solar light in electric energy with high efficiency is the perovskite with lead halide, whose chemical formula is CH_{3}NH_{3}PbI_{3}. The photovoltaic cell have 5 fundamental layers: perovskite layer, which is between an electron transport layer (ETL) and hole transport layer (HTL), layers are empty space without electrons. These 3 layers are between two electrodes, a metal and a transparent oxide conductor.

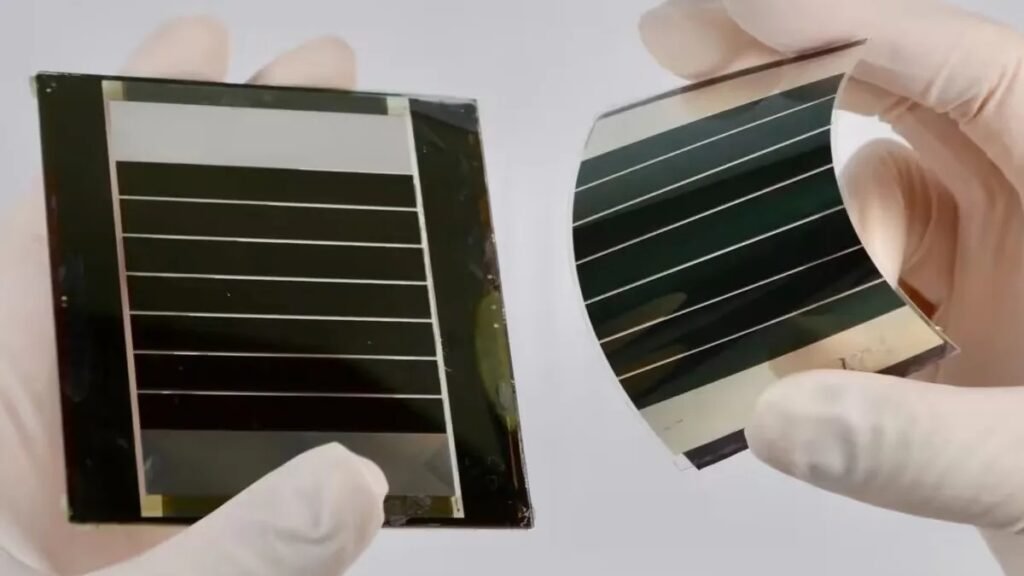
Advantages and challenges
Perovskite solar cells have advantages such as:
- An energetic efficiency higher than 25%, more efficient than photovoltaic cells based in other materials currently used.
- Lower production cost, due to material’s abundance and production method.
- Photovoltaic cells can be flexible.
However, still exist some challenges that need to be solved:
- Perovskite solar cells have lead, a dangerous element for the environment and human health.
- Low stability at ambient conditions: exposition to moisture, oxygen, heat and mechanical stress considerably reduce this cell’s life span.
- Currently, they are made with a method for low scale, it’s necessary to develop a large scale manufacturing method.
In addition to this, perovskite materials can serve for manufacturing of sensors, LEDs, lasers, catalyzers for fuel cells, non volatile memories, etc.


