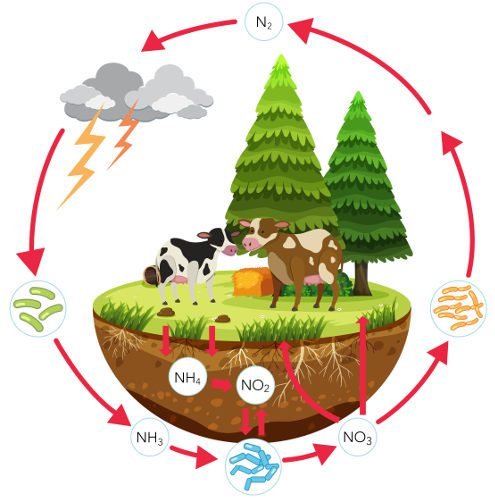
This post subject is the nitrogen cycle, an important geochemical process for the survival of living beings inland and aquatic ecosystems.
Nitrogen cycle’s stages
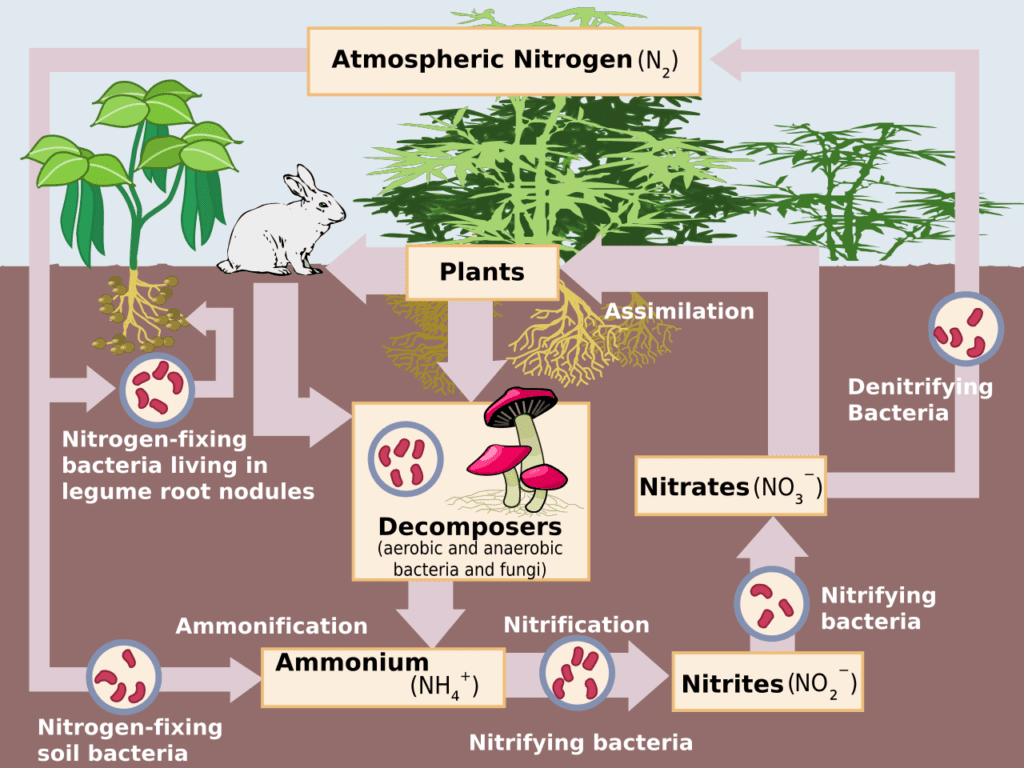
Fixation
The nitrogen gas (N_{2}) compose 78% of air. However, plants and animals can directly absorb nitrogen from air, because it’s little reactive. In this stage, prokaryotes (without cell nucleus) bacteria convert the gas in ammonia (NH_{3}), making the following chemical reaction.
N_{2}+16ATP+8e^{-}+8H^{+}\rightarrow 2NH_{3}+H_{2}+16ADP+16Pi
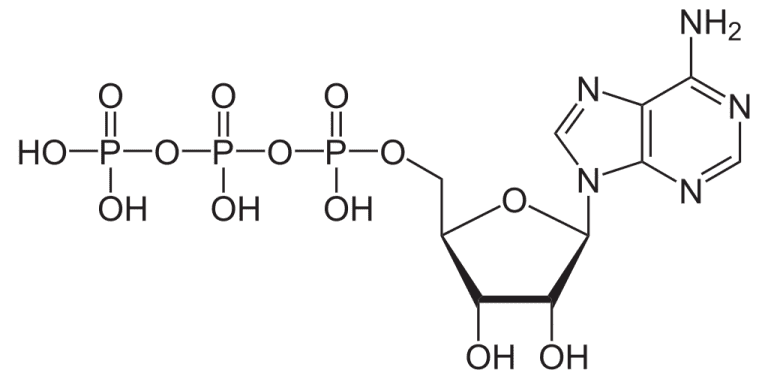
ATP is the adenosine triphosphate, an organic molecule that stores energy. ADP is adenosine diphosphate, one of products when ATP releases energy, along with inorganic phosphate, represented by Pi.
The fixation process is made using the nitrogenase enzyme. Some bacteria like the Azotobacter and Clostridium are free in the soil, while the Rhizobium lives on the root leguminous plants.

The conversion of nitrogen in ammonia also can be made by electric sparks and lightning.
Ammonification
The nitrogen also can come from remains of animals and plants and waste. The aminals absorb nitrogen eating the plants. Decomposing organisms like saprophytic bacteria and fungal species consume organic material and liberate ammonia. When it combines with water, ammonium hydroxide (NH_{4}OH) is formed. When its dissoved on water, produce the ammonium (NH_{4}^{+}) and hidroxide (OH^{-}) ions.
NH_{3}+H_{2}O\rightarrow NH_{4}^{+}+OH^{-}
Part of ammonia is used by plants to synthesize amino acids, blocks that form the proteins.
2NH_{3}+2H_{2}O+4CO_{2}\rightarrow 2CH_{2}NH_{2}COOH+3O_{2}
Nitrification
Most part of produced ammonia in the previous stage is converted in nitrite (NO_{2}^{-}) by prokaryotes bacteria Nitrosomonas e Nitrosococus.
2NH_{3}+3O_{2}\rightarrow 2NO_{2}^{-}+2H^{+}+2H_{2}O+energia
Ammonium ion also can be converted in nitrite by the microorganisms.
2NH_{4}^{+}+3O_{2}\rightarrow 2NO_{2}^{-}+4H^{+}+2H_{2}O
Then, Nitrobacter bacteria convert nitrite in nitrate (NO_{3}^{-}).
2NO_{2}^{-}+O_{2}\rightarrow 2NO_{3}^{-}+energia
Nitrite production requires more oxygen than nitrate. Therefore, the Nitrosomonas and Nitrosococcus bacteria are in the most aerated part of the soil, while the Nitrobacter are in the part with less oxygen. These micro-organisms use energy generated in these reactions to produce organic substances from carbon dioxide (CO_{2}) and water (H_{2}O).
6CO_{2}+12H_{2}O+energia\rightarrow C_{6}H_{12}O_{6}+6O_{2}+6H_{2}O
This process is called chemosynthesis and does not need solar light. The nitrate is absorbed by plants to produce organic compounds, like enzymes, chlorophyll, amino acids and nucleic acids (DNA and RNA).
Denitrification
In this stage, heterotrophic bacteria, which don’t produce their own food, consume organic matter and release nitrogen back to the atmosphere.
5C_{6}H_{12}O_{6}+24NO_{3}^{-}+24H^{+}\rightarrow 30CO_{2}+42H_{2}O+12N_{2}+energia
The nitrogen cycle in an aquatic environment
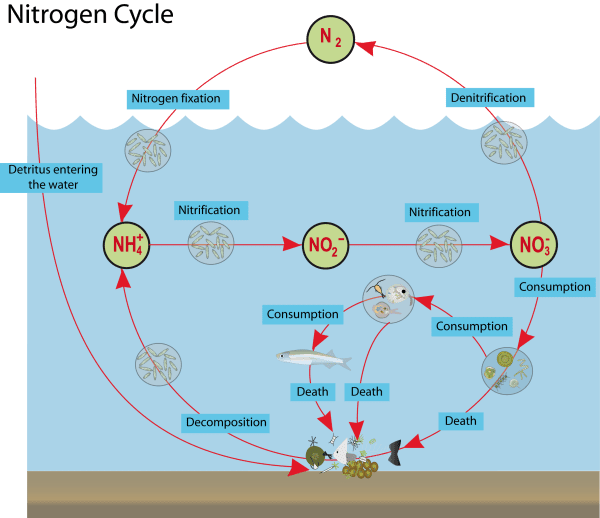
In seas and oceans, nitrogen fixation from air is made by marine bacteria and cyanobacteria, also known as cyanophyta or blue-green algae. Despite the names, thay aren’t either algae, either bacteria. Other marine bacteria make the other stages.

Compounds that have nitrogen goes to the bottom of the sea, forming sedimentary rocks. Eventually, geological uplift bring sedimentary rocks to dry land. The rocks’ weariness and meteorological actions allow plants to use the contained nitrogen.
Eutrophication
It’s an unbalance caused by excess of organic material on water, can have natural or antropic (human action) causes. When agricultural fertilizers are overused, this excess go to rivers, lakes and groundwater. Fertilizer are rich in nitrogen and phosphorus, causing the accelerated growth of plants. Consequently, increase the number of algae and aerobic bacteria, which consume oxygen, causing the death of other living beings due to low level of oxygen.

In the next post of Environment, will be explained what is pH and it’s influence on nitrogen cycle.